This block is the only one having all three types of elements: metals, nonmetals, and metalloids. The p-block elements can be described on a group-by-group basis as the group 13 called as the icosagens, the group 14 as the crystallogens, the group 15 as the pnictogens, the group 16 as the chalcogens, the group 17 as the halogens and the group 18 as the noble gases. Alternatively, the p-block can be described as containing the post-transition metals, the metalloids and the reactive nonmetals including the halogens and the noble gases.
The corrosive nonmetals of the p-block (F, Cl, Br, and I) tend to form ionic compounds with metals whereas the remaining reactive nonmetals tend to form covalent compounds. The metalloids (B, Si, Ge, As, Sb, Te, and At) tend to form either covalent compounds or alloys with metals.
- Boron Family (Icosagens)
The elements in the Group 13 of the periodic table are known as the Boron family or the Group III or the Icosagens. This group is comprising of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and the chemically non-characterized nihonium (Nh). Each of the elements in this group has 3 electrons in its outer shell in which an isolated, neutral group 13 atom has the s² p¹ configuration in the ground state. Most of its members are poor metals, except for boron. Boron is a semi-metal. The rest of the elements with the possible exception of nihonium, are soft metals and are also considered as the post-transition metals. The elements in the boron group are characterized by having three valence electrons.


Boron, a brittle hard black semi-metal, that occurs sparsely from the bombardment by the subatomic particles produced from natural radioactivity. Aluminium, a common soft silvery metal that occurs widely on earth and the third most abundant element in the Earth's crust (8.3%). Gallium, an easily melted silvery metal that is found in the earth with an abundance of 13 ppm. Indium, a soft silvery metal which is the 61st most abundant element in the earth's crust, and thallium, a highly toxic very soft metal which is found in moderate amounts throughout the planet. Nihonium, an unstable man-made element which is never found in nature and therefore is termed a synthetic element.
- Carbon Family (Crystallogens)
The group of the elements in the Group 14 in the periodic table is also known as the Carbon family or the Group IV or the Crystallogens. This group is comprising of Carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). Each of the elements in this group has 4 electrons in its outer shell in which an isolated, neutral group 14 atom has the s² p² configuration in the ground state.
![68 [PDF] GROUP 5 PERIODIC TABLE NAME PRINTABLE HD DOWNLOAD ZIP](https://blogger.googleusercontent.com/img/proxy/AVvXsEg0Un8vMlSEjG6kGCaq64eKu-MqM47oB10ORztzaJR4cS16pbn7Nha6GXJARsFdfENeZdt4y4-QEOnwyu_KUttjUl-4_VlYPXOJWXB_9R2Vf2NlZ3F-1u-bEzZwSZ7P43olkTXrBxrbbtuQJxcIKPyRVTVX8bpkve7M=s0-d)
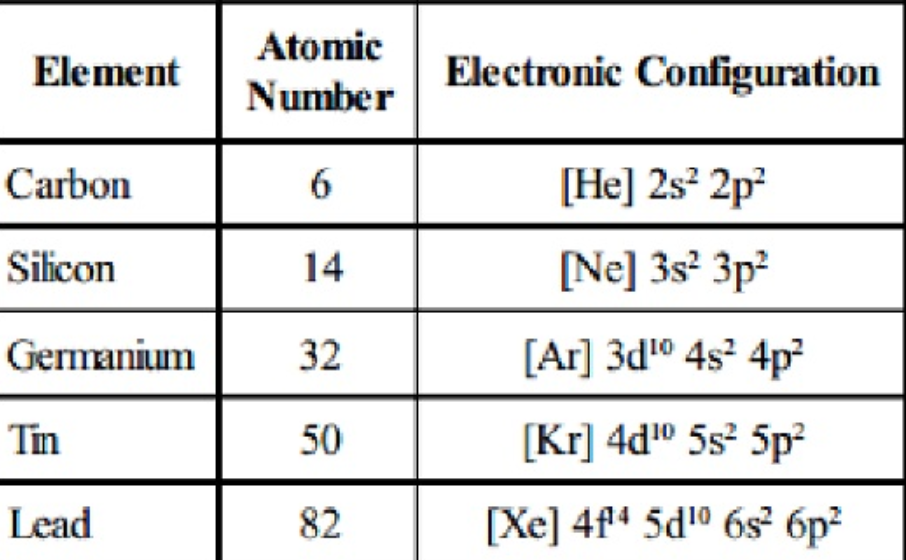
These elements, especially carbon and silicon, have a strong tendency to form covalent bonding with other metals, which usually brings the outer shell to eight electrons. Only carbon forms negative ions (anions), in the form of carbide (C⁴⁻) ions. Silicon and germanium are semi-metals (metalloids), each can form +4 ions. . Tin and lead are poor metals and they can form +4 and +2 oxidation states. Flerovium, a synthetic radioactive element that its half life is very short which is not enough to see its properties but it is still most likely a post-transition metal.
- Nitrogen Family (Pnictogens)
The group of the elements in the Group 15 in the periodic table is also known as the Nitrogen family or the Group V or the Pnictogens. This group is comprising of nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and the chemically non-characterized synthetic element moscovium (Mc). Each of the elements in this group has 5 electrons in its outer shell in which an isolated, neutral group 15 atom has the s² p³ configuration in the ground state, that is, 2 electrons in the s sub-shell and 3 unpaired electrons in the p sub-shell.

Nitrogen is a colorless non-reactive gas and the most important elements of this group which is the principal component of Earth's atmosphere. Phosphorus is a reactive white or red solid nonmetal which is essential to all known forms of life. Arsenic is a highly toxic semi-metal. Antimony is also a semi-metal. Bismuth is a metal. They all have 5 valence electrons. Most of them form +5 or -3 chemical compounds. Moscovium is a synthetic and an extremely radioactive chemical element and it has a half-life of only 0.65 seconds, but still mostly like to be a post-transition metal.

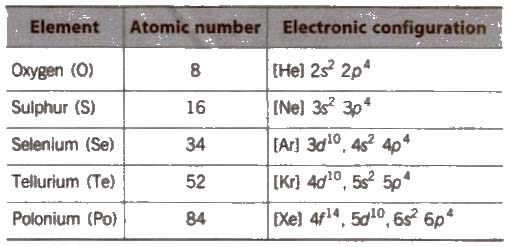
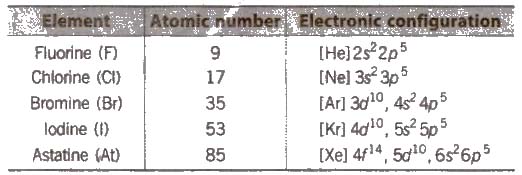
- Oxygen Family (Chalcogens)
The group of the elements in the Group 16 in the periodic table is also known as the Oxygen family or the Group VI or the Chalcogens. This group is comprising of oxygen (O), sulfur (S), selenium (Se), tellurium (Te), the radioactive element polonium (Po) and the chemically non-characterized synthetic element livermorium (Lv). Each of the elements in this group has 6 electrons in its outer shell in which an isolated, neutral group 16 atom has the s² p⁴ configuration in the ground state, that is, 2 electrons in the s sub-shell and 4 unpaired electrons in the p sub-shell. Their most common oxidation states are −2, +2, +4, and +6.


The first three elements (oxygen, sulfur and selenium) are nonmetals and the last two (tellurium and polonium) are semi-metals. All of the naturally occurring chalcogens have some role in biological functions, either as a nutrient or a toxin. Oxygen is a colorless gas generally obtained by separation of air into nitrogen and oxygen. The primary use of elemental oxygen is in steel-making. Sulfur is extracted from oil and natural gas which is mostly converted into sulfuric acid, which is heavily used in the chemical industry. Selenium and tellurium are produced as byproducts of copper refining. Selenium's most common application is glass-making. Tellurium compounds are mostly used in optical disks, electronic devices, and solar cells. Polonium and livermorium are most available in particle accelerators. Polonium is radioactive Some of polonium's applications are due to its radioactivity. Livermorium is a synthetic and an extremely radioactive chemical element with a half-life of about 60 milliseconds, but still mostly like to be a post-transition metal.
- Flourine Family (Halogens)
The group of the elements in the Group 17 in the periodic table is also known as the Fluorine family or the Group VII or the Halogens. This group is comprising of fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At) and the artificially created element tennessine (Ts). Each of the elements in this group has 7 electrons in its outer shell in which an isolated, neutral group 17 atom has the s² p⁵ configuration in the ground state, that is, 2 electrons in the s sub-shell and 5 unpaired electrons in the p subshell. Their most common oxidation states are −1.
Due to the fact that they are missing one electron, they are very reactive, so they are likely to join with other elements on the left side of the periodic table to make compounds. When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. Hence, the "halogen" are also known as "salt-producer". All of the halogens form acids when bonded to hydrogen.

They are rarely found alone in nature, except astatine, which is not found in nature. Fluorine is the most reactive, while iodine is the least reactive. Astatine is very radioactive and hard to get. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure.
- Noble Gases
The group of the elements in the Group 18 in the periodic table is also known as the Group VIII or the Noble Gases. This group is comprising of helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), the radioactive radon (Rn) and the artificially created element Oganesson (Og). Each of the elements except helium in this group has fully filled 8 electrons in its outer shell in which an isolated, neutral group 18 atom has the s² p⁶ configuration in the ground state, that is, 2 electrons in the s sub-shell and 6 electrons fully accommodated in the p sub-shell. Hence, all of them are monoatomic, meaning each molecule is a single atom. They almost never react with other elements.The noble gases are a group of elements that are all gases. All of these gases are found in air. They make up around 0.96% of the atmosphere. Noble gas compounds can be formed from noble gases.

Noble gases were discovered by Lord Rayleigh and Sir William Ramsay. Rayleigh won the Nobel Prize in Physics in 1904 for his work on noble gas.[2] Ramsay won the Nobel Prize in Chemistry in 1904 for his work with noble gas. When the noble gases are used in cold cathode tubes to produce light, each of them has a different color. Since Radon is radioactive, it is usually not used for lighting. Oganesson is a synthetic chemical element with a half life of 0.89 ms. It has the highest atomic number and highest atomic mass of all known elements. Here are pictures of what the others look like:


Noble gases were discovered by Lord Rayleigh and Sir William Ramsay. Rayleigh won the Nobel Prize in Physics in 1904 for his work on noble gas.[2] Ramsay won the Nobel Prize in Chemistry in 1904 for his work with noble gas. When the noble gases are used in cold cathode tubes to produce light, each of them has a different color. Since Radon is radioactive, it is usually not used for lighting. Oganesson is a synthetic chemical element with a half life of 0.89 ms. It has the highest atomic number and highest atomic mass of all known elements. Here are pictures of what the others look like:



Comments
Post a Comment