
Atoms of chemically pure elements may bond to each other chemically in more than one way, allowing the pure element to exist in multiple chemical structures and they are known as allotropes, which may cause any differences in their properties. Allotropes are different structural modifications of an element in which the atoms of the element are bonded together in a different manner The ability of an element to naturally exist in more than one structural forms is known as 'allotropy' which is also the property of some chemical elements to exist in two or more different forms, in the same physical state. The term allotropy is used for elements only, not for compounds. The more general term, used for any crystalline material, is polymorphism. Allotropy refers only to different forms of an element within the same phase
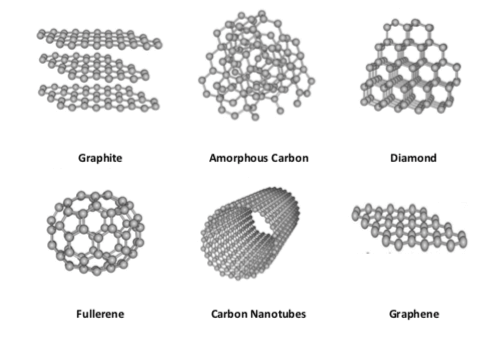
For example, carbon can be found as diamond, graphite, graphene, fullerene and carbon tube. Diamond, which has a tetrahedral structure around each carbon atom whereas graphite, which has layers of carbon atoms with a hexagonal structure stacked on top of each other, in which the graphene is a single layer of graphite that is very strong, but the fullerenes have nearly spherical shapes and carbon nano-tubes, which are tubes with a hexagonal structure. Even though these are made of only kind of atom, they may differ from each other in electrical properties due to the difference in the spatial arrangement of atoms.
The standard state, also known as the reference state, of an element is defined as its thermodynamically most stable state at a pressure of 1 bar and a given optimum temperature (typically at 298.15 K). In thermo-chemistry, an element is defined to have an enthalpy of formation of zero in its standard state. For example, the reference state for carbon is graphite, because the structure of graphite is more stable than that of the other allotropes.
For some elements, allotropes have different molecular formulae despite difference in phase; for example, two allotropes of oxygen (di-oxygen O₂ and ozone O₃) both can exist in the solid, liquid and gaseous states.
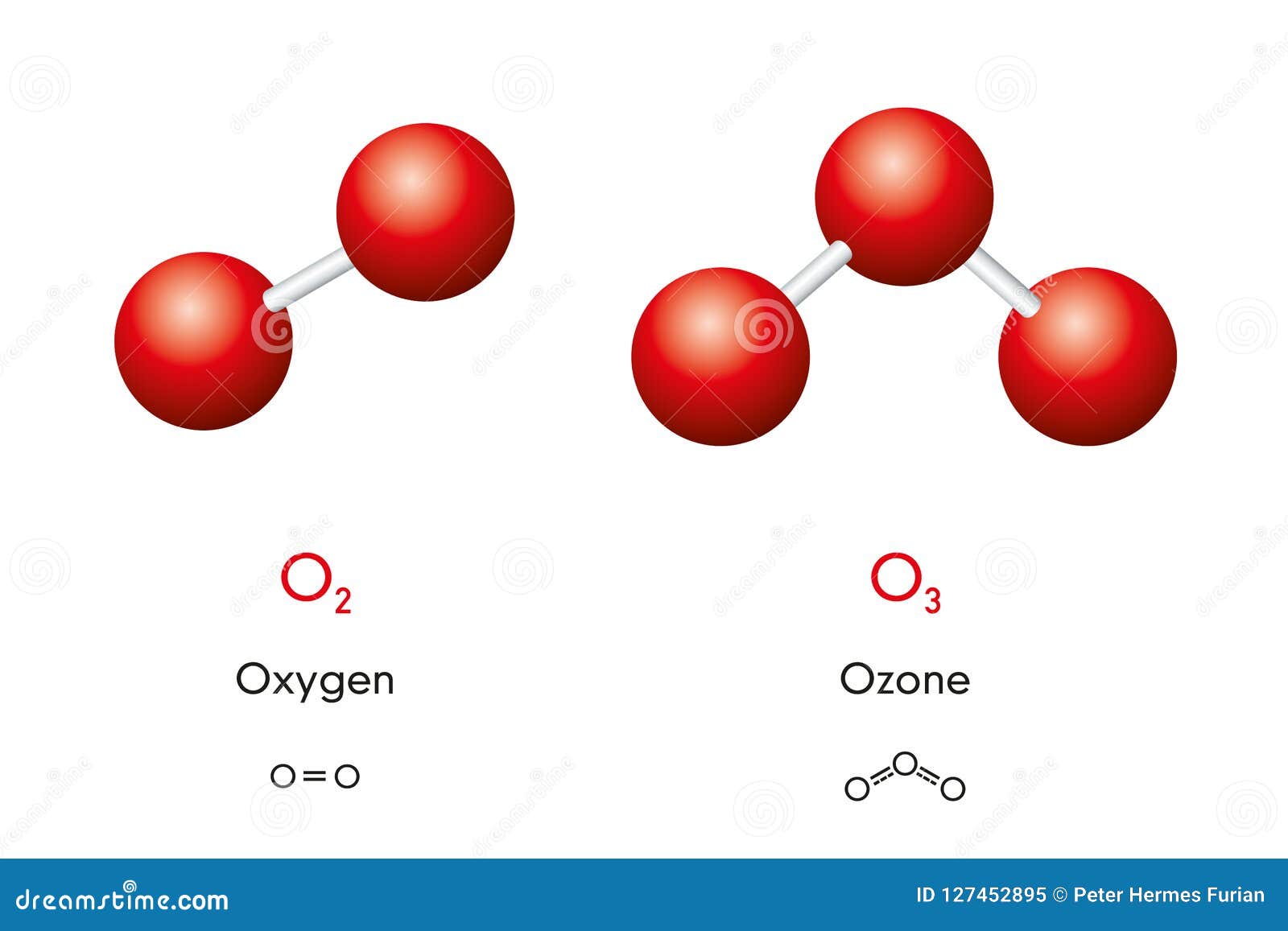

For some elements, allotropes have different molecular formulae despite difference in phase; for example, two allotropes of oxygen (di-oxygen O₂ and ozone O₃) both can exist in the solid, liquid and gaseous states.

The element sulfur exists as many allotropes. In terms of large number of allotropes, sulfur is second only to carbon.

 |
| Monoclinic Sulphur |
 |
| Rhombic Sulphur |
Comments
Post a Comment