In our daily life, every ordinary matter we experiencing are baryonic in nature, that is, they all made up of baryonics sub-particles such as protons, neutrons and electrons which are perfectly assembled in a particular pattern to form atoms.
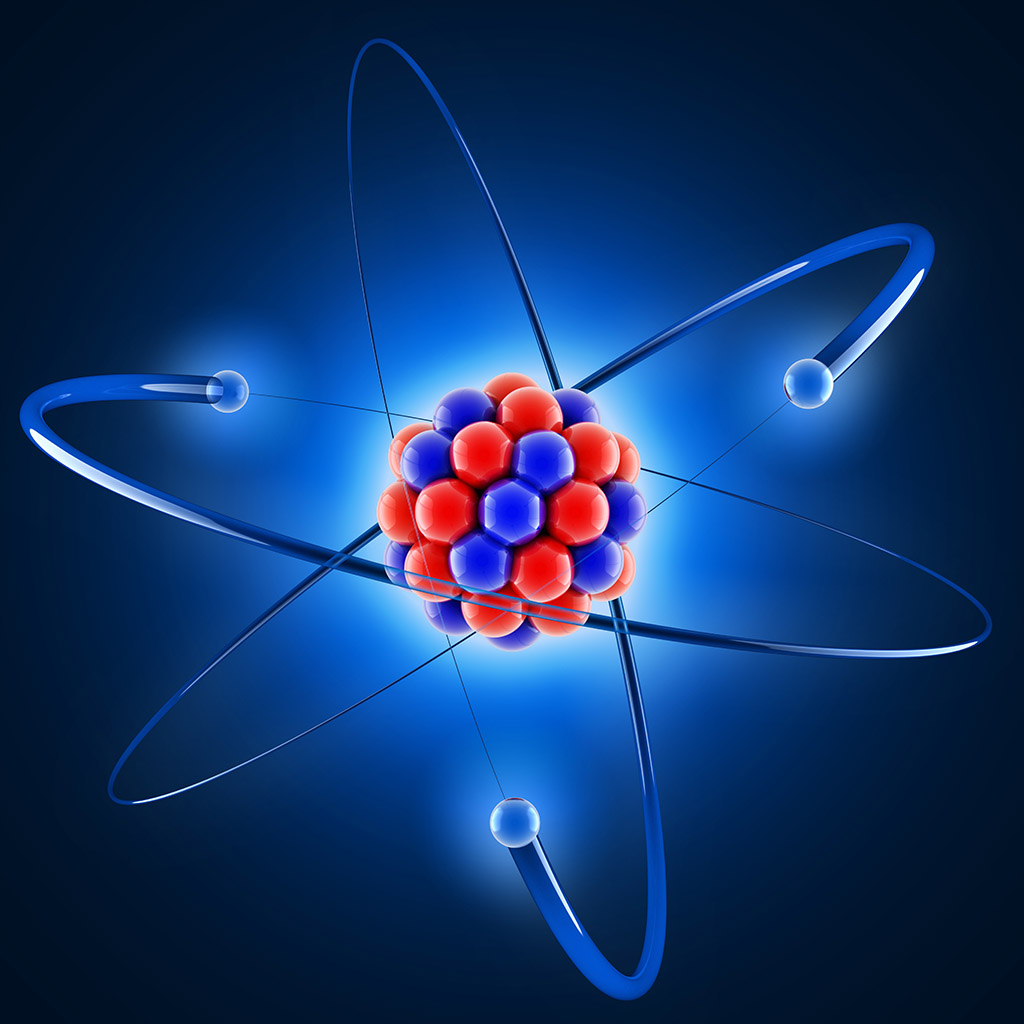
The atom is the basic unit of matter which is the smallest thing that can have a chemical property. Atoms are made up of two types of hadron particles; the protons (positively charged particles), the neutrons (particles with no charge) and one type of lepton particles called the electrons (negatively charged particles).The protons and the neutrons are heavier and remain stay at the middle of the atom as a nucleus of the atom. The nucleus is surrounded by a cloud of electrons which are very lightweight. The electrons have the negative charge and the nucleus have the positive charge, so they attract each other and the electrons orbit or travel around the nucleus by the electromagnetic force.
But everything we sees, feels are not made by only single type of atom, all are composed of different types of atoms. Each type of atoms indicate each elements. Every elements are differ from its type of atoms which has unique number of protons in its nucleus or the number of electrons revolving around its nucleus.
But everything we sees, feels are not made by only single type of atom, all are composed of different types of atoms. Each type of atoms indicate each elements. Every elements are differ from its type of atoms which has unique number of protons in its nucleus or the number of electrons revolving around its nucleus.

Comments
Post a Comment