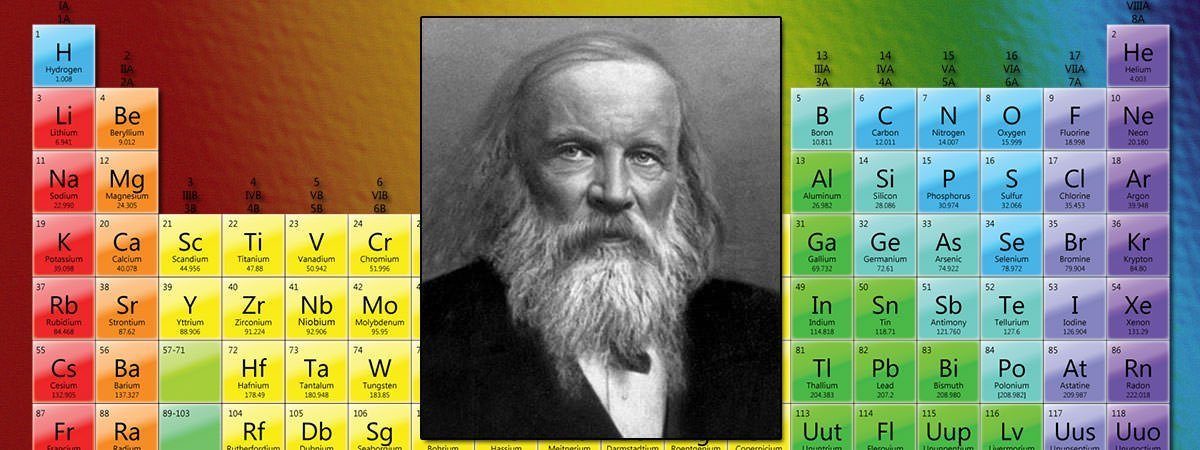
The quest for a systematic arrangement of the elements started with the discovery of individual elements. The organization of the periodic table can be used to derive relationships between the various element properties, and also to predict chemical properties and behaviors of undiscovered or newly synthesized elements. Many scientists made significant contributions that eventually Mendeleev to construct his table.
Russian chemist Dmitri Mendeleev published the first recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Most of his forecasts proved to be correct. The periodic table did not end with Mendeleev but continued to take shape for the next 150 years.
The Periodic Table is an excellent tool for looking at the elements and the key to using it is to understand the code of its structure. Using the boxes, columns and rows will help you to learn about the properties of elements. The modern periodic table now provides a useful framework for analyzing chemical reactions, and continues to be widely used in chemistry, nuclear physics and other sciences. The Periodic Table as we know it today is not the fruit of the labor of a single man, rather a collective effort of some of the most brilliant minds ever.
Comments
Post a Comment