
A chemical element is a pure substance that contains only one type of atom. If a substance contains more than one type of atom, it is a compound. An element can be a solid, liquid or gas.
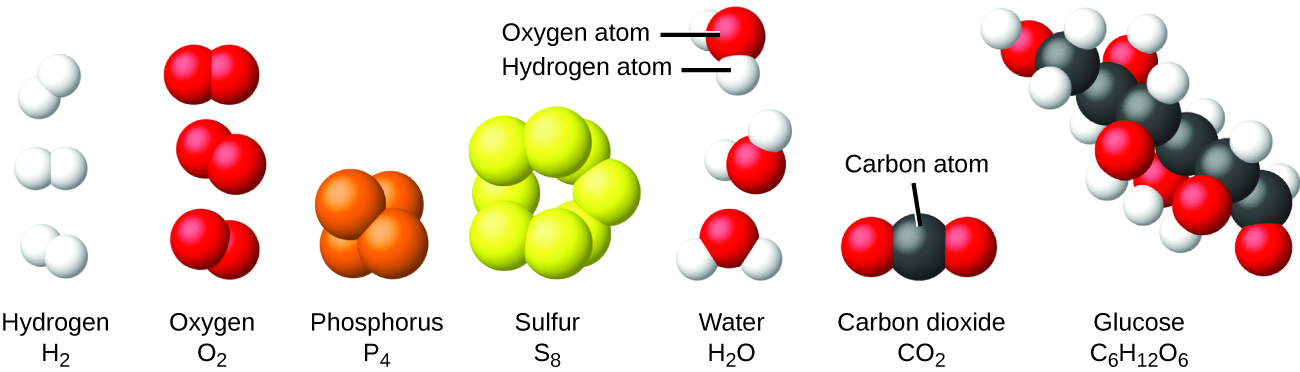
The smallest particle of such an element is an atom. Atoms are made up of protons, neutrons, and electrons. Each element contains only one kind of atom. Each kind of atoms are identified by its atomic number. The Atomic Number of an atom is the number of protons present in the nucleus of the same atom. The number of protons in the nucleus causes its electric charge. This fixes the number of electrons in its normal (unionized) state. The electrons in their atomic orbitals determine the atom's various chemical properties. The total number of neutrons and the protons in an atomic nucleus of an atom is the Atomic Mass of the same atom.

Elements are the basic building blocks for all types of substances. When they are combined with each other, they can form molecules.
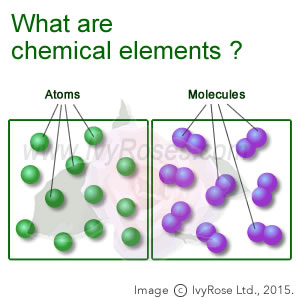
118 different chemical elements are known to modern chemistry. 92 of these elements can be found in nature and the others can only be made in laboratories. The two lightest elements, hydrogen and helium, were mostly formed in the Big Bang and are the most common elements in the universe. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust. The human body is made up of 26 elements. The last natural element discovered was uranium, in 1789. The first man-made element was technetium, in 1937. Chemical elements are commonly arranged in the periodic table. Where the elements are on the table tells us about their properties relative to the other elements.

118 different chemical elements are known to modern chemistry. 92 of these elements can be found in nature and the others can only be made in laboratories. The two lightest elements, hydrogen and helium, were mostly formed in the Big Bang and are the most common elements in the universe. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust. The human body is made up of 26 elements. The last natural element discovered was uranium, in 1789. The first man-made element was technetium, in 1937. Chemical elements are commonly arranged in the periodic table. Where the elements are on the table tells us about their properties relative to the other elements.
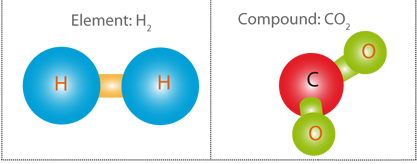

When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals such as copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur.

While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these gets mix together in any proportion to form new structures. Such new structures are not compounds, but they occur as mixtures or, when the elements are metals, alloys. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.


While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these gets mix together in any proportion to form new structures. Such new structures are not compounds, but they occur as mixtures or, when the elements are metals, alloys. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.

Comments
Post a Comment