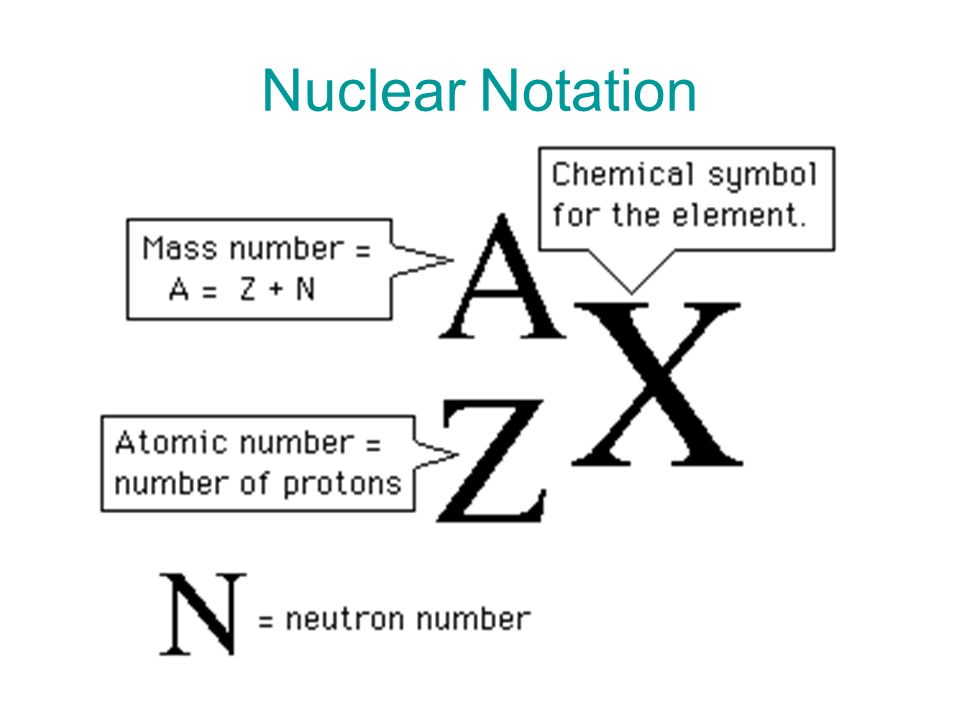
Nuclear notation is used to specify a particular element with its chemical symbol to indicate the atomic mass number and the atomic number of the same element. It is also known as 'AZX Notation' because 'A' is the atomic mass number (number of nucleons) with a superscript at the upper left of the chemical symbol, 'Z' is the atomic number with a subscript at the lower left and 'X' is used to indicate the chemical symbol.
Chemical elements are also given a unique chemical symbol. Chemical symbols of elements come from their English or Latin names. For example, carbon has the chemical symbol 'C', and sodium has chemical symbol 'Na', after the Latin natrium. Tungsten is called 'W' after its German name, wolfram. 'Au' is the symbol for gold and it comes from the Latin word for gold, aurum. Another symbol which comes from Latin is 'Ag'. This is the element silver and it comes from the Latin argentum. Lead's symbol, 'Pb', comes from the Latin plumbum. Some more recently discovered elements were named after famous people, like einsteinium, which was named after Albert Einstein.
For Example: the Nuclear Notation of A Carbon Atom as shown below
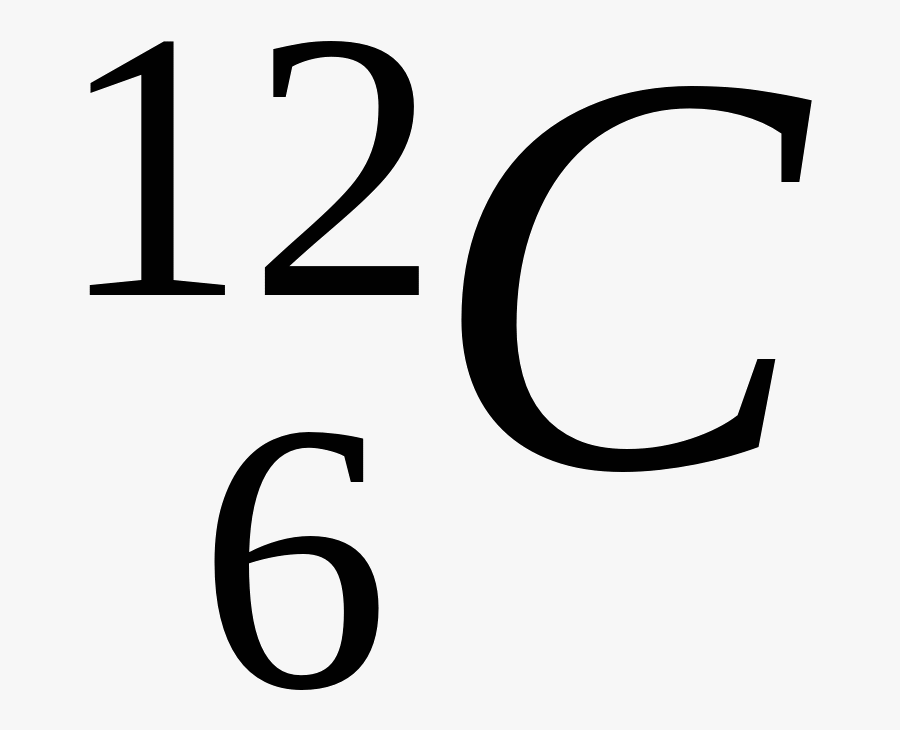
For Example: the Nuclear Notation of A Carbon Atom as shown below
Comments
Post a Comment